|
Laboratory for Energy Materials and Nano-Biomedicine IN DEPARTMENT OF CHEMICAL AND MATERIALS ENGINEERING |
|
|
|
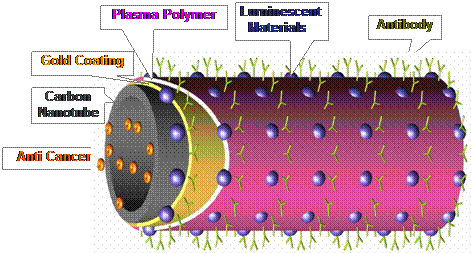
Research Activities in Nano Biomedicine A critical challenge for biomarking, based on luminescent materials, has been the development of nano structures with highly visible or characteristic near infrared emissions for precise imaging, combined with nanoscale cavities for drug storage and delivery. The design of such materials requires complex nanostructures that have both the intense emissions and appropriate storage geometries. We report here a scheme for the use of novel nanostructures designed to satisfy both of these important requirements. Hypodermal and in vivo organic imaging from quantum dot conjugated carbon nanotubes has been realized in mice for the first time. The coupling of quantum dots on carbon nanotubes was materialized based on a novel plasma nanotube surface polymerization. The quantum dot activated carbon nanotubes exhibited intense visible light emissions in both fluorescent spectroscopy and in vivo imaging. These experimental results can be extended to the development of new techniques for early cancer diagnosis. There is an increasing need for the early detection of cancer, prior to the detection of anatomic anomalies. Thus, a major challenge in cancer diagnosis is to locally biomark cancer cells for maximum therapeutic benefit 1, 2. In cancer therapy, the targeting and localized delivery of drugs are also key challenges. One promising strategy for overcoming these challenges is to make use of highly luminescent nanoparticles for qualitative or quantitative in vitro detection of tumor cells 3-8. Recently, in vivo imaging using single wall carbon nanotubes has also been reported 9, 10 In the cancer diagnosis and treatment by nanotechnology, it has been critical to develop novel nanostructures that combine multiple functionalities including biocompatibility, fluorescent signaling, drug storage and delivery, and coupling to biological molecules such as DNA/RNA, antibodies, and viruses. In the design of such a complex nanostructure, nanotubes of a variety of materials may serve as ideal substrates, on which multi functional structures needed for biomedical diagnosis and treatment can be developed. Based on above considerations, we report a novel nanotube surface design ideally suited for this purpose. In contrast to previous studies, this novel nano system was materialized via several unique synthesis routs including plasma surface functionalization. An idealized representation of the nano scale design for a luminescent carbon nanotube is shown schematically in Fig. 1. The hollow center of the nanotube can be filled with a cancer treatment drugs. The outer surface of the nanotube is coated with luminescent materials for enhanced optical properties. Although carbon nanotubes are known to have near-infrared emissions, the luminescence intensities are generally too weak for whole-body in vivo imaging. Therefore, intense emissions and tunable wave lengths can only be provided by luminescent materials such as quantum dots (QDs). Compared with other traditional, rare-earth-doped semiconductors11 and organic fluorophores12, QDs have superior properties, including higher quantum yield with a broader emission spectrum than that of rare earth phosphors. In addition, the spectra are much sharper than the emission spectra of organic fluorophores3-9. QDs are semiconducting nanocrystals that possess size-tunable electronic and optical properties resulting from quantum confinement effects13, 14. They offer high resistance to photo-bleaching, thus making them attractive materials for optoelectronics15, 16 and in vivo biosensing applications17. After the chemical treatment, the QDs go into the lighter aqueous phase. This ensures the synthesis of water-soluble QDs with functional groups, such as QD-NH2 and QD-COOH. Due to the chemical inertness of carbon nanotubes, their surface modification must be completed in order to attach the QDs. For instance, the acid oxidation of carbon nanotubes, resulting in the carboxyl groups at the tips and other high defect density sites was one of the effective approaches 18. The direct covalent functionalization was based on the reaction between carboxyl and amine. In this study a unique plasma coating method was developed in order to generate the carboxyl function groups on multi-wall carbon nanotubes (MWCNTs-COOH) for covalent coupling to CdSe/ZnS quantum dots. These quantum dots were functionalized with amine (QD-NH2) 19-23. The MWCNTs were chosen for their larger inner wall diameters (50-80 nm), which are more suitable for drug storage. The toxicity and bio-compatibility of carbon nanotubes have been previously studied 24, 25. After successfully demonstrating the concept of nano-biomarker using MWCNTs, other more biodegradable substrates maybe selected for the same application. It must be noted that this paper focuses on the surface functionalization of MWCNTs based on a new nano scale surface design, uniquely suited for biomedical diagnosis and treatment. A novel surface functionalization method was employed to uniformly deposit ultra thin films of functional groups on MWCNTs in order to attach luminescent materials on their surfaces. The purpose of in vivo imaging experiments in this research was to demonstrate the feasibility of the quantum dots activated MWCNTs in biomedical diagnosis. For the purpose of representing the complete design, as shown in Fig. 1, we have described other possible functions of the novel surface nano structures such as drug storage and attachment of antibodies. However, these tasks are currently being pursed, and the results are not presented in this study. Thus, the present research is primarily centered on the design of the surface nanostructures, development of luminescent carbon nanotubes, and their corresponding physical properties. EviTags 600 CdSe/ZnS quantum dots (emission wavelength of 600 nm) with hydrodynamic diameters of 40 nm were purchased from Evident Technologies. They were functionalized with amine and kept in water with the concentration of core quantum dot at 2.6 (nmols/ml). Commercial grade multi-wall carbon nanotubes (MWCNTs) were provided by Applied Science Inc. as substrates in this experiment. These MWCNTs were 70-150 nm in diameter and several microns in length. In this experiment, the surfaces of MWCNTs were treated first by super-acid (HNO3 + H2SO4). A thin film (< 5 nm) was then deposited by plasma polymerization onto their surfaces in order to provide the carboxyl functional groups. Plasma polymerization is a novel and effective method for surface functionalization of nanotubes and nanoparticles 19-23. The main principle of the plasma polymerization is that the ionized and excited monomer molecules created by the electrical field bombard and react on the surface of the substrate. These activated molecules may etch, sputter, or deposit on the substrate surface. Due to these characteristics, the plasma technique can be used for polymer surface functionalization on various nanotubes and nanoparticles. The experimental procedures and conditions for plasma surface functionalization and characterization have been previously published 19-23. The plasma reactor for surface functionalization consists mainly of a radio frequency source, the glass vacuum chamber, and press gauge. The monomers were intro?duced from the gas inlet during the plasma polymerization. In this study, acrylic acid (AA) monomer was used for the surface functionalization of the nanotubes.
Fig. 1 Schematic diagram showing the design of the nanostructures.
References 1. Walt, D. R. Miniature Analytical Methods for Medical Diagnostics. Science. 308, 217-219 (2005). 2. Ferrari M., Cancer Nanotechnology: Opportunities and Challenges. Nature Reviews Cancer. 5, 161-171 (2005). 3. Gao, X., Cui, Y., Levenson, R. M. Chung, L. W. K. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969-976 (2004). 4. Dubertret, B., Skourides, P., Norris, D. J., Noireaux, V., Brivanlou, A. H. & Libchaber, A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science. 298, 1759-1762 (2002). 5. Wu, X., Liu, H., Liu, J., Haley, K. N., Treadway, J. A., Larson, J. P., Ge, N., Peale, F. & Bruchez, M. P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 21, 41-46 (2003). 6. Jaiswal, J. K., Mattoussi, H., Mauro, J. M. & Simon, S. M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 21, 47-51 (2003). 7. Larson, D. R., Zipfel, W. R., Williams, R. M., Clark, S. W., Bruchez, M. P., Wise, F. W. & Webb, W. W. Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo. Science. 300, 1434-1436 (2003). 8. Derfus, A. M, Chan, W. C. W. & Bhatia, S. N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 4, 11-18 (2004). 9. Cherukuri, P., Gannon, C. J., Leeuw, T. K., Schmidt, H. K., Smalley, R. E., Curley, S. A., and Weisman, R. B. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence, PNAS, 103, 18882-18886 (2006) 10. Liu, Z., Cai, W., He, L, Nakayama, N., Chen, K., Sun, X., Chen, X., and Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice, Nat. Nanotech. 10.1038 (2006) 11. Chen, X. Y., Yang, L., Cook, R. E., Skanthakumar, S., Shi, D. & Liu, G. K. Crystallization, phase transition and optical properties of the rare-earth-doped nanophosphors synthesized by chemical deposition. NanoTech. 14, 670-674 (2003). 12. Väisänen, V., Härmä, H., Lilja, H. & Bjartell, A. Time-resolved fluorescence imaging for quantitative histochemistry using lanthanide chelates in nanoparticles and conjugated to monoclonal antibodies. Luminescence. 15, 389- 397 (2000). 13. Brus, L. Quantum crystallites and nonlinear optics. Appl. Phys. A, 53, 465-474 (1991). 14. Alivisatos, A. P. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. 100, 13226-13239 (1996). 15. Banerjee, S. & Wong, S. S. Synthesis and characterization of carbon nanotube-nanocrystal heterostructures. Nano Lett. 2, 195-200 (2002). 16. Haremza, J. M., Hahn, M. A. & Krauss, T. D. Attachment of single CdSe nanocrystals to individual single-walled carbon nanotubes. Nano Lett. 2, 1253-1258 (2002). 17. Chan, W. C. W. & Nie, S. M. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 281, 2016-2018 (1998). 18. Rinzler, A. G. et al. Large-scale purification of single-wall carbon nanotubes: process, product, and characterization. Appl. Phys. A. 67, 29-37 (1998). 19. Shi, D., He P., Lian J., Wang L. M., Mast D. & Schulz M. Plasma deposition of ultrathin polymer films on carbon nanotubes. Appl. Phys. Lett. 81, 5216-5218 (2002). 20. Shi, D., Wang, S. X., van Ooij, W. J., Wang, L. M., Zhao, J. G., & Yu, Z. Uniform Deposition of ultrathin polymer films on the surface of alumin nanoparticles by a plasma treatment. Appl. Phys. Lett., 78, 1243-1245 (2001). 21. Shi, D., He, P., Lian, J., Wang, L. M., Mast, D. & Schulz, M. Plasma coating of carbon nanotubes for enhanced dispersion and interfacial bonding in polymer composites. Appl. Phys. Lett., 83, 5301-5303 (2003) 22. Shi, D. L., He, P., Lian, J., Wang, L. M., Van Ooij, W. J., Plasma Deposition and Characterization of Acrylic Acid Thin Film on ZnO Nanoparticles. J. of Mat Res. 17, 2555 (2002). 23. He, P., Shi, D., Lian, J., Wang, L. M., Ewing, R. C., Van Ooij, W. J., Li, Z., Ren, Z. F., Plasma Deposition of Thin Carbonfluorine Films on Aligned Carbon Nanotubes, Appl. Phys. Lett., 86, 043107 (2005). 24. Jia, G., Wang, H., Yan, L., Wang, X., Pei, R., Yan, T., Zhao, Y., and Guo, X., Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol., 39, 1378 (2005). 25. Lam, C. W., James, J. T., McCluskey, R. & Hunter, R. L. Pulmonary toxicity of single-wall Carbon nanotubes in mice 7 and 90 Days after intratracheal instillation. Toxicol Sci., 77, 126-134 (2004). 26. Shaw, C. F. III, Gold-Based Therapeutic Agents, Chem. Rev., 99, 2589-2600 (1999) 27. Shi, D., Lian, J., Wang, W., Liu, G. K., He, P., Dong, Z., Wang, L. M., & Ewing, R. C. Luminescent nanoparticles by surface functionalization, Adv. Mat., 18, 189-193 (2006). 28. Wang, W., Shi, D., Lian, J., Liu, G. K., He P., Dong Z., Wang L. M., & Ewing R. C. Luminescent Hydroxylapatite Nanoparticles by Surface Functionalization, Appl. Phys. Lett., 89, 183106 (2006).
|
|
RESEARCH INFO
|
|
|
RELATED LINKS
|
|