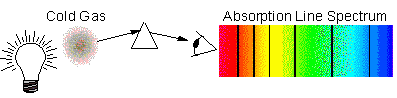Pulling it all Together: Black Body and Atomic Transitions
Intensity changes smoothly with wavelength and all colors are present. The photons are created by electrons moving with energy proportional to the heat, and no specific transition is observed. This is what the spectrum of a SOLID 'Black Body' (such as the very hot element at the center of a light bulb) looks like:
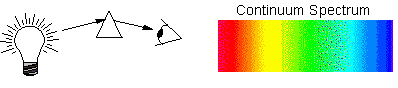
Kirchoff's Law 2: Emission-Line Spectrum
The light is emitted only at a few
particular wavelengths, while most of the spectrum is dark. This is typical
of hot, tenuous gas (such as in a neon-tube). Here, the individual atomic transitions are seen:
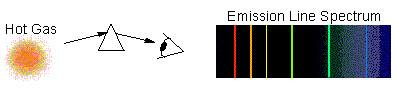
Kirchoff's Law 3: Absorption-Line Spectrum
Here, we see a continuum spectrum, but
it is missing discrete wavelengths, making them appear dark in the spectrum.
This is what occurs when a cool, tenuous gas resides in front of a bright
continuum (solid, Black Body) source: