Atomic Identification Using Spectral Lines
Hydrogen atoms have a completely different set of orbits than other elements (Helium, Oxygen, Nitrogen, etc). By studying the Energy Level Diagram of different kinds of atoms, we can learn to identify unique sets of lines which identify a specific atom.

Note that when the electron is in the n=1 level, it is then in the ground state. This electron has the minimum amount of energy possible.
To identify the atoms found inside a hot glowing gas (such as a neon-like tube), we would use a prism to make a SPECTRUM, and look at the wavelength of the individual lines emitted. By looking for certain lines and patterns of lines, we can identify the atom present within the gas.
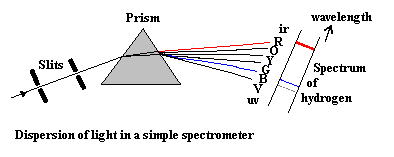
For a tutorial on spectral lines, go HERE or just to see examples of spectra go HERE.