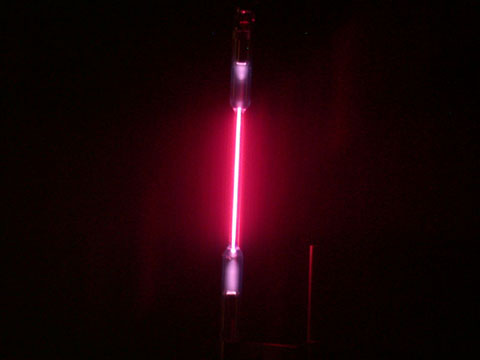
Not every light source emits light of all colors like an incandescent light bulb. Consider the hydrogen bulb shown below.

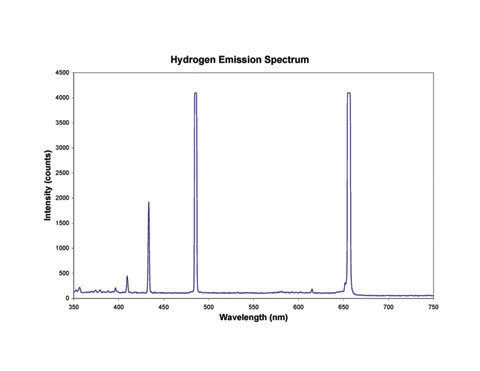
The spectrum obtained by passing this light through a prism or grating is show in the next image. Note that instead of containing all the colors of the rainbow, it contains a series of lines of various colors. Each color is located at the same position in the picture as it was in the continuous spectrum, but most of the image is dark because hydrogen emits light of only a few colors. This is called a line spectrum or a discrete spectrum because of the individual, discrete, bands of color.

A graph of the intensity of light vs. wavelength (shown below) confirms what we see in the spectrum. The light is very intense at some wavelengths, and there is none at others. Each peak corresponds to one of the colors in the image above. In fact, chemists use a graph like this to determine the wavelength of the lines in a discrete spectrum. For an explanation of why the hydrogen spectrum looks like this, see the tutorial on Atomic Spectra.
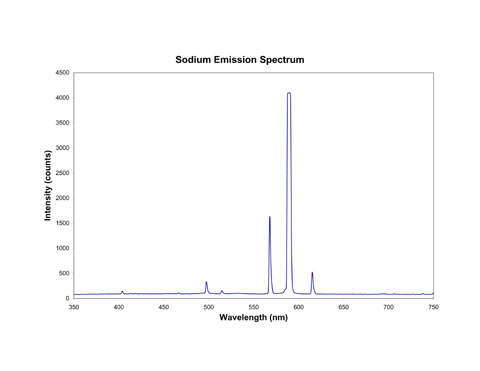
The orange-yellow glow of street lights comes from the line spectrum of sodium in the lamps. As you can see below, there is a particularly bright line at 589 nm which gives sodium lights their color. (It is actually a pair of closely-spaced lines, but the resolution of the camera isn't good enough to separate the two lines, so they look like one.)


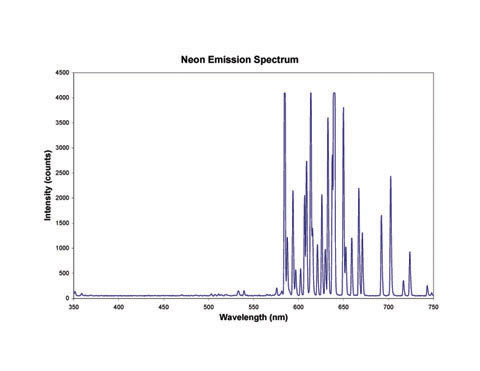
Neon lights, on the other hand, contain a lot of lines in the red region of the spectrum as you can see in the images and graph below. In fact, if you see a light that looks like a neon light but it isn't red, it probably doesn't contain any neon at all!


| Contents | Previous | Next |