TPR and Nuclear Transport
 Dokudovskaya et al. 2002
Dokudovskaya et al. 2002
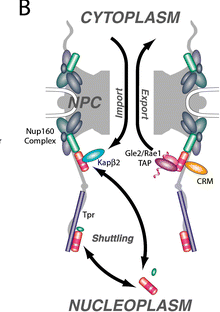
Tpr (translocated promoter region) is a 270 kD protein whose exact function in nuclear transport is unknown. The N-terminal domain of Tpr is an α-helical coiled coil, and the C-terminal region is a highly acidic non-coiled domain. It has been shown that Tpr localizes to the nuclear basket of the NPC and it has been observed that in some cell types (for example, amphibian) it forms filaments that extend into the interior of the nucleus (Cordes et al. 1997).
Several domains of Tpr have been identified, including the nuclear localization signal and a region of the protein that is sufficient for association with the NPC. It is hypothesized that Tpr has a role in mRNA export because overexpression of Tpr results in accumulation of poly(A)+ RNA in the nucleus (Bangs et al. 1998). In Xenopus, importin ß coimmunoprecipitates with Tpr (Shah et al. 1998). These observations suggest that Tpr may have a function in nuclear transport, architecture of the nuclear pore complex, and possibly the formation of tracks for transport to and from the NPC (Bangs et al. 1998).
Mlp1p is a homolog of Tpr in Saccharomyces cerevisiae, and another protein Mlp2p is closely related to Mlp1p. Mlp1p has been shown to be associated with the nuclear envelope and the nuclear pore complexes of S. cerevisiae (Strambio de-Castillia et al. 1999). Although single knockouts of MLP1 or MLP2 do not cause an observable effect on nuclear transport, the double knockout is impaired in nuclear import. Overexpression of Mlp1p causes formation of large structures within the nucleus that displace chromatin. The C-terminal region of Mlp1p, like that of Tpr, is free and may interact with other factors (Strambio de-Castillia et al. 1999). These observations suggest that Mlp1p may have a role in nuclear transport and connecting the NPC with the nuclear interior.
Proposal:
Hypothesis: Certain domains of Mlp1p and Tpr are required for nuclear transport through direct or indirect interactions with the cargo protein and its associated carrier proteins.
What are the domains of Mlp1p that are important in the mediation of protein transport? Are these regions in Tpr involved in nuclear transport?
In order to define the role of these proteins in nuclear import, what are the proteins that each interacts with?