1. What domain(s) of Mlp1p is/are essential for nuclear transport?
It has been observed that in the double knockout mlp1Δmlp2Δ, the kinetics of nuclear import are disrupted. This can be measured in an assay described in Strambio-de-Castillia et al., 1999. The single mutant mlp2Δ does not have an observable nuclear transport phenotype. Therefore, it will be tested whether mutant Mlp1p with selected domains deleted will rescue the nuclear transport defect of the double mutant.
Constructs: The nuclear localization sequence (1496-1565) is known, so this sequence will not be deleted, because the localization of the protein should be as it is in the wildtype strain. Deletions starting at the 3' end of the genomic DNA will be made, and this DNA will be ligated into a CEN vector. These constructs will express C-terminal truncation mutants when transformed into yeast.
Strains transformed:
Negative control: mlp1Δmlp2Δ + empty vector
Positive Control: mlp1Δmlp2Δ + wildtype MLP1 construct
Experimental: mlp1Δmlp2Δ + mutant constructs
Before carrying out the kinetic assay, the cellular localization of the protein will be monitored by fixing cells and then probing with antibodies against Mlp1p. The antibody used in Strambio-de-Castillia et al., 1999 recognizes an epitope in the C-terminal region of the protein. Therefore, other antibodies will be needed to detect the proteins in which this region is deleted. Secondary antibodies with a fluorophore attached will allow the visualization of the Mlp1p protein. A negative control with nonspecific antibody as the primary antibody should be used in order to establish the amount of background fluorescence should be used. The double knockout plus the wildtype MLP1 construct would act as a positive control. Only constructs in which the Mlp1p is localized to the nuclear envelope and the nuclear pore complexes as it is in the positive control will be used in the kinetic assay.
The assay is carried out as follows:
Each of the above strains must also be transformed with a construct that will express an NLS-GFP protein. Logarithmically growing yeast are poisoned (with deoxyglucose and sodium azide) to block the production of energy. This causes the active transport of NLS-GFP into the nucleus to be reduced, and the signal eventually becomes equal in the cytoplasm and nucleus. When the metabolic inhibitors are removed, the rate of NLS-GFP import into the nucleus can be measured. The percentage of nuclear GFP signal is plotted vs. time, as shown in the graph below
.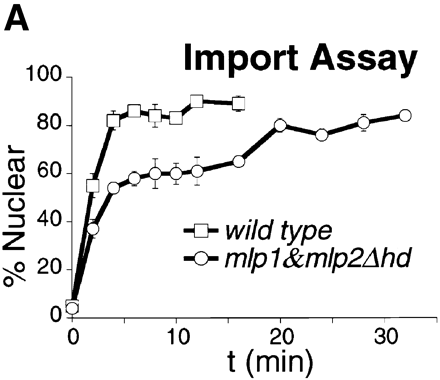
If the experimental constructs show kinetics similar to the negative control or show slower kinetics compared to the positive control, then that region deleted in the construct is somehow involved in nuclear transport.
(Strambio-de-Castillia et al. 1999)