Mechanism of Myosin V
Mechanism for kinesin
Motors at work
In
general, motor proteins consist of a head and two tails. The head binds to the
organelle to be transported while the tails binds to microtubule/microfilament .
Hydrolysis of one ATP causes a conformational change which causes
the molecule to "take a step" forward, cycle is repeated and
the molecule "walks" down its
track.
Myosin V and Kinesin
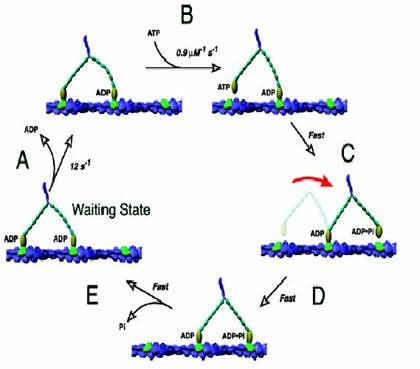
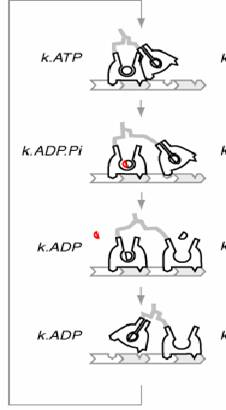
ADP release from the trailing head is followed by ATP binding which causes dissociation of the trailing head leading to the formation of a one head bound intermediate state. The leading head then executes a swing of its lever arm, which throws the trailing head in front and the new leading head then executes a diffusional search and rebinds the 13th actin filament (in case of myosin) and a tubulin dimer in Kinesin. Step size for Myosin is 37nm while kinesin takes smaller steps of 8nm.

Mechanism of Myosin V

Mechanism for kinesin
Kinesin is the molecular motor, which pulls things towards the outer reaches of the cell from the negative (-) end of the microtubule towards the positive (+) end of the microtubule.
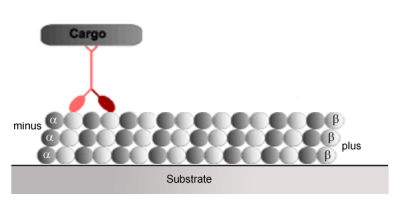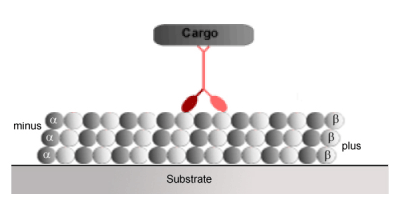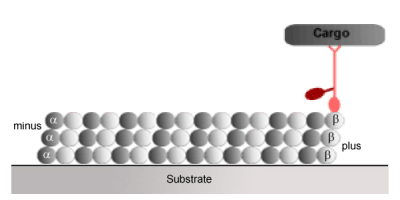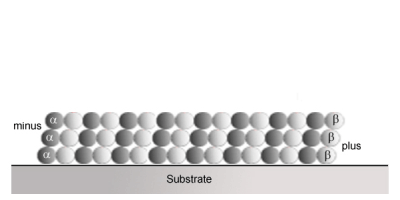
http://www.imb-jena.de/~kboehm/Kinesin.html
Dynein
Dynein, binds to the microtubule and uses the energy in ATP molecules to move from the positive (+) end of the microtubule where new tubulin dimers are adding to the microtubule towards the minus (-) end of the microtubule. Each small step, requires the hydrolysis of one ATP molecule. Dynein pulls subcellular materials toward the center of the cell, or in the case of mitosis, toward the poles of the spindle and thus toward the centers of the two new daughter cells.
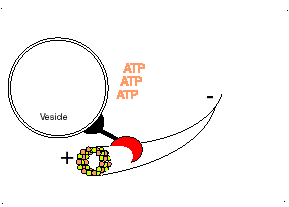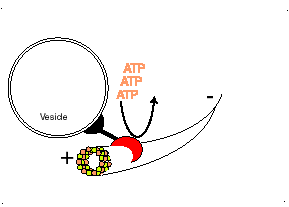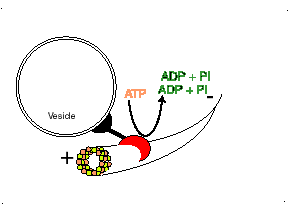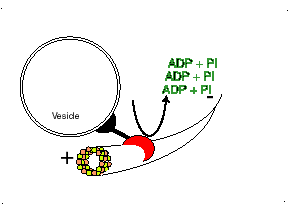
http://bio.winona.msus.edu/berg/ANIMTNS/Dynein.htm
Motor
protein involvement in intracellular
transport can be mimicked in vitro by
Motility Assays.
Home Introduction Transport Systems Types of Motor Proteins Motors at Work Motility Assays Questions