Motility Assays
Motility
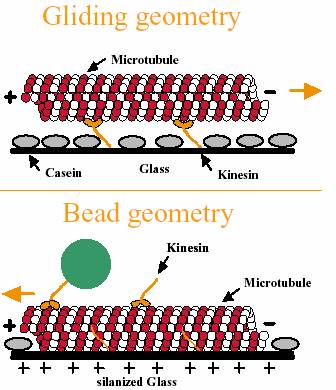
Assays used to study motor use two different geometries, called the bead assay
and the gliding assay. In the bead assay the microtubules/microfilaments are
fixed to the surface and the respective motors move on them. A bead is bound to
one or more motors to allow visualization or the exertion of force in an optical
trap. In a gliding assay, the tails of the motors move the
microfilaments/microtubules across the surface.

The
experimental set up for the gliding assay involves four basic steps:
1)adsorbing
a protein monolayer onto the surface by flushing in a solution containing casein
or albumin to reduce denaturation
2)adsorbing
the molecular motors to the penetrated surface by exchanging solutions
3)adsorbing
the microtubules/microfilaments to the motors from a third solution
4)imaging
motility with optical microscopy
In
the bead assay, motors and filaments/ microtubules are interchanged in steps (2)
and (3)
Flow
cells
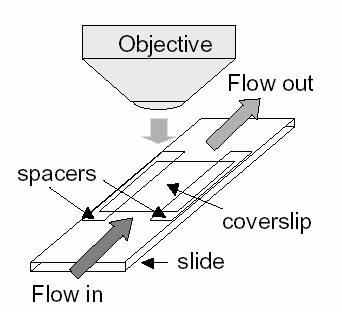
A
simple flow cell of a classic motility assay allows easy exchange of solutions,
illumination with light and a fluorescence microscope to be used which will aid
in visualization.
Home Introduction Transport Systems Types of Motor Proteins Motors at Work Motility Assays Questions