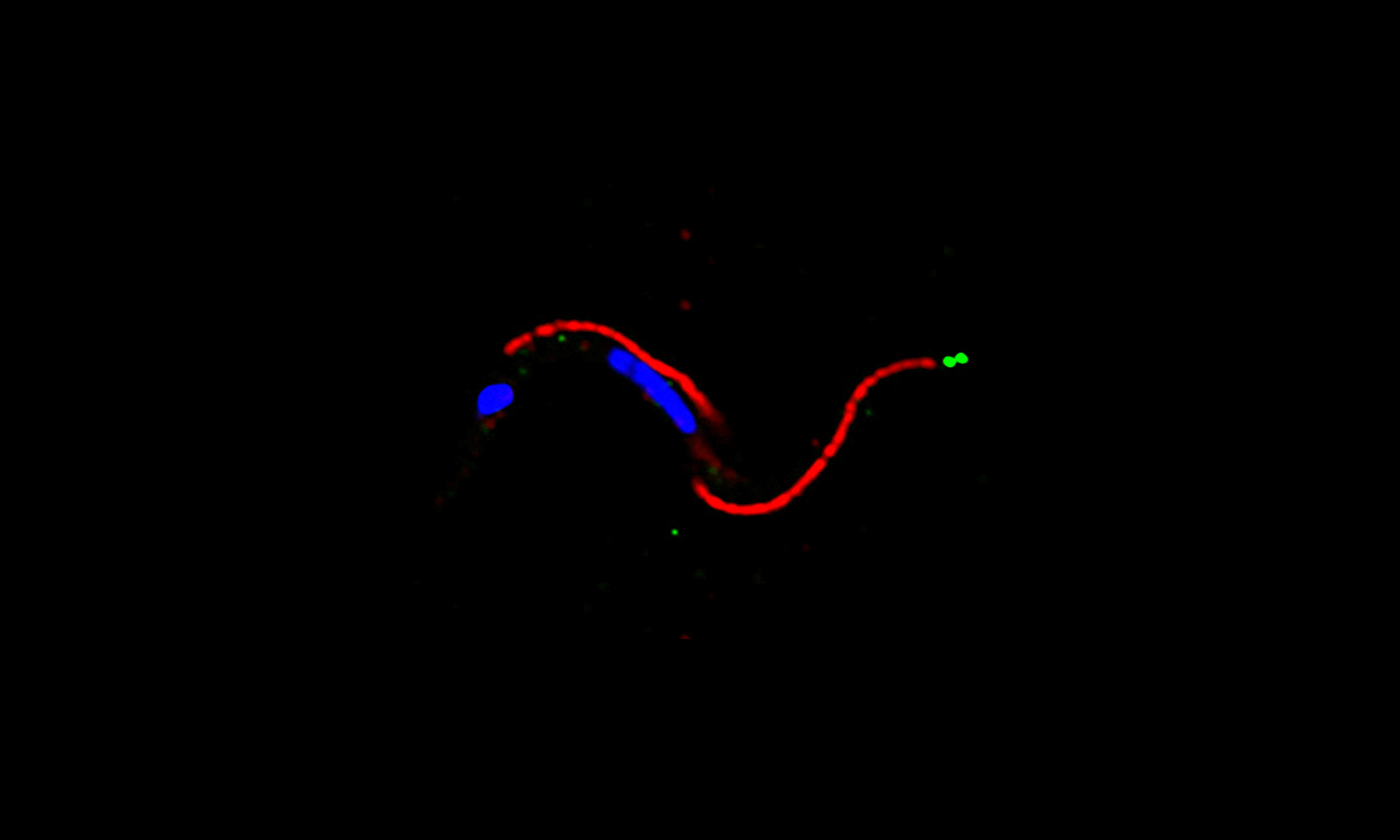
Research in my laboratory focuses on the study of signal transduction pathways in trypanosomes, flagellated protozoan parasites that belong to the Kinetoplastida order. Different species of trypanosomes infect a variety of vertebrates, including animals and humans, and produce fatal diseases like sleeping sickness, caused by Trypanosoma brucei, and Chagas disease, caused by Trypanosoma cruzi.
Chagas disease is a life-threatening sickness affecting 6 to 7 million people worldwide. There is no vaccine to prevent the disease or satisfactory treatment available. T. cruzi parasites are mainly transmitted to humans by contact with feces/urine of infected triatomine bugs. Oral infections by consumption of food contaminated with vector feces are also common. An estimated 300,000 people are currently infected in the United States, from which 45,000 exhibit cardiomyopathy, while most cases remain undiagnosed and untreated. Currently, there are only two accepted drugs to treat Chagas disease. Besides their adverse side effects, the efficacy of these drugs decreases the longer a person has been infected. Understanding T. cruzi biology is a key component to develop alternative strategies to diagnose and treat Chagas disease patients.
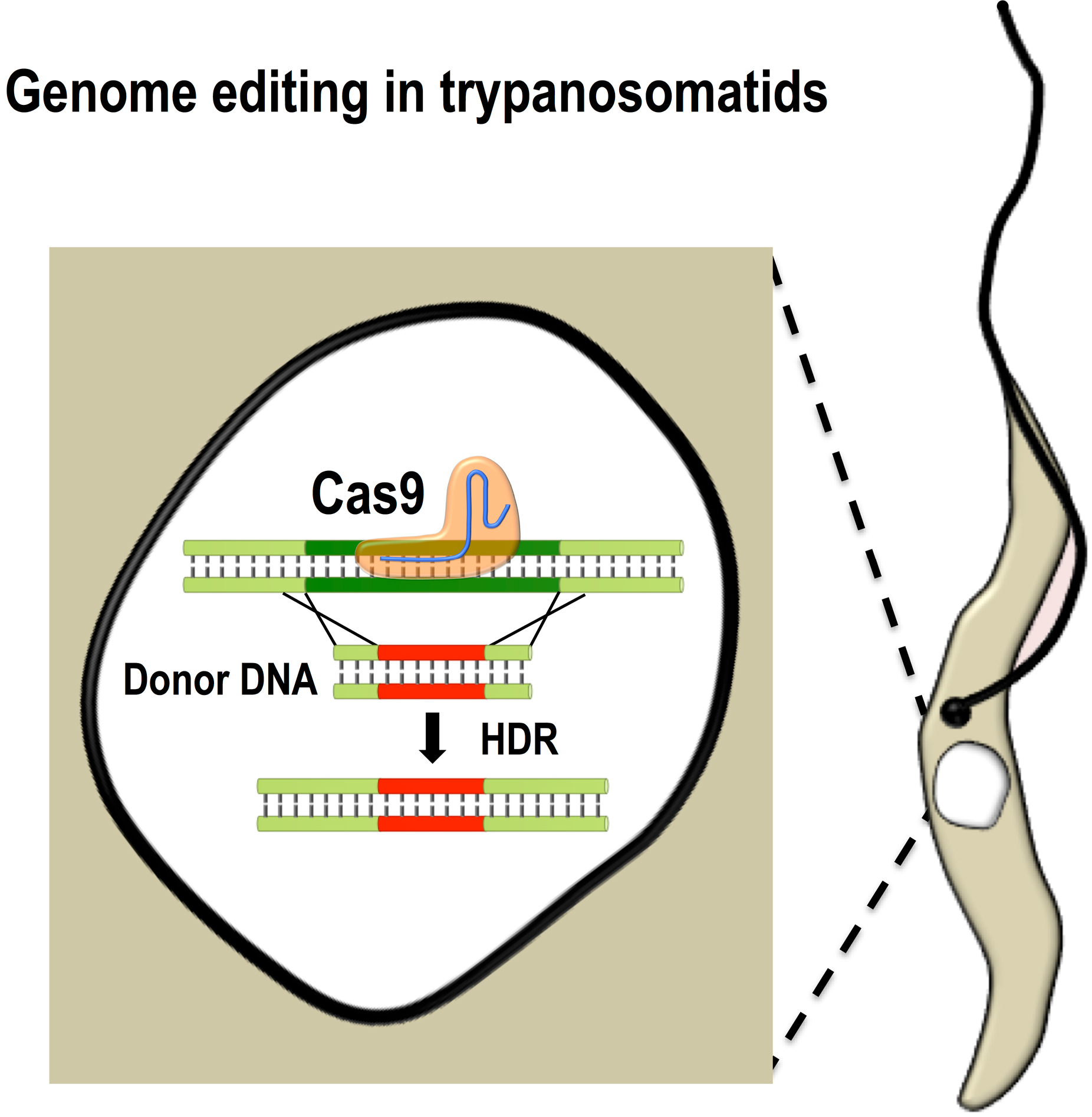
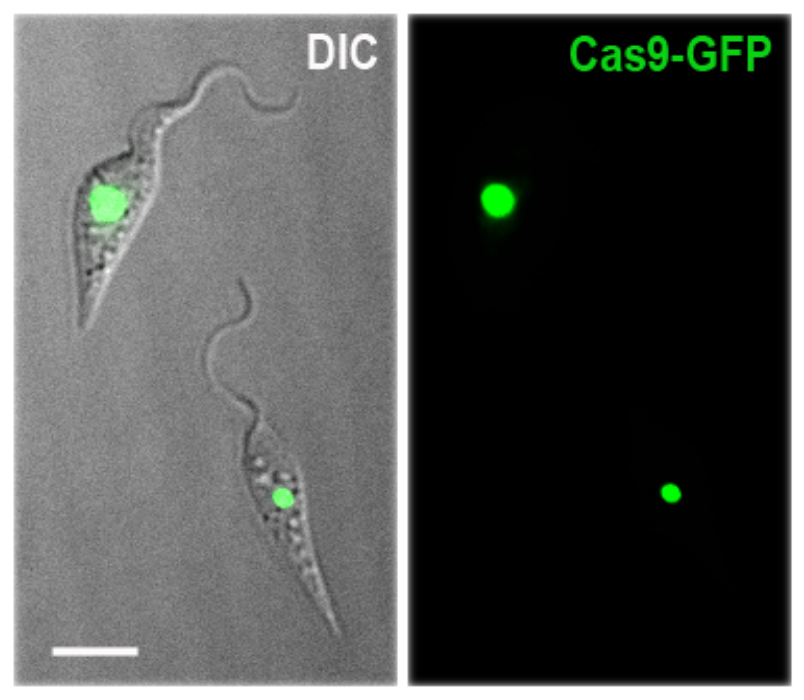
In addition to the importance of T. cruzi for human health, this parasite exhibits unique metabolic and structural characteristics that make it an attractive model of early divergent eukaryotic cells. The main stages of T. cruzi life cycle can be maintained in laboratory conditions and infection of tissue cultured host cells can be systematically performed. As a postdoctoral trainee one of my main contributions to science was the development of the methodology to perform genome editing in T. cruzi by CRISPR/Cas9. Using this system, we are now able to generate null mutants and perform endogenous C-terminal tagging, gene complementation and in situ mutagenesis in T. cruzi, which represents a tremendous improvement in the genetic manipulation of this parasite. In my laboratory, we combine genetic and biochemical approaches to dissect the specific function of proteins involved in calcium and cAMP signaling pathways in T. cruzi.
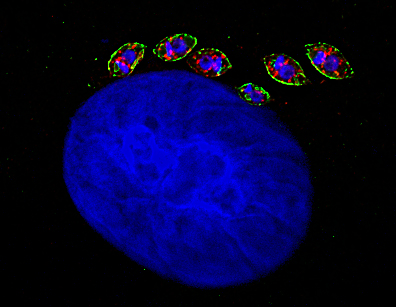
Calcium ion is an important second messenger in trypanosomatids that regulates a vast repertoire of cellular processes, including cell motility, host cell invasion, differentiation, osmoregulation and cell bioenergetics. Several proteins involved in calcium homeostasis have been identified in trypanosomes. However, the T. cruzi genome contains a wide variety of calcium-interacting uncharacterized proteins. 3′,5′-cyclic AMP (cAMP) is another universal second messenger that mediates cell differentiation in T. cruzi. However, this signaling pathway is poorly understood in trypanosomes. During its life cycle T. cruzi alternates between a vertebrate host and an insect vector. To survive drastic environmental changes T. cruzi differentiates into four main developmental stages. How does the parasite sense those changes, what type of stimulus triggers specific cellular responses that lead to cell differentiation, and what is the role of the main proteins involved in calcium and cAMP signal transduction pathways, are some of the questions we pursue to answer.
Lander Lab News
April 2024

Congratulations to Josh Carlson who received the 2024 J. Robie Vestal Award for Outstanding Masters Student in the Department of Biological Sciences
March 2024
Congratulations to Milad Ahmed for receiving the University Research Council’s Graduate Student Stipend and Research Cost Program for Faculty-Student Collaboration, which will cover his full stipend and laboratory supplies over the summer:
August 2023
Our most recent research work is now in the news: