|
The normal cellular
copy of the prion protein has been found in retinal cells, myeloid cells
besides neurons. The normal cellular prion protein is a membrane bound
Glycoprotein with a Glycerophosphatidyl anchor. This prion protein in its
normal cellular form is believed to be involved in several functions in the
cell. Some of these functions include protection against antioxidant
activity by regulating copper ion concentration; neuroprotective networks
via signal transduction through Tyrosine kinases and may even prevent
apoptosis in retinal cells.
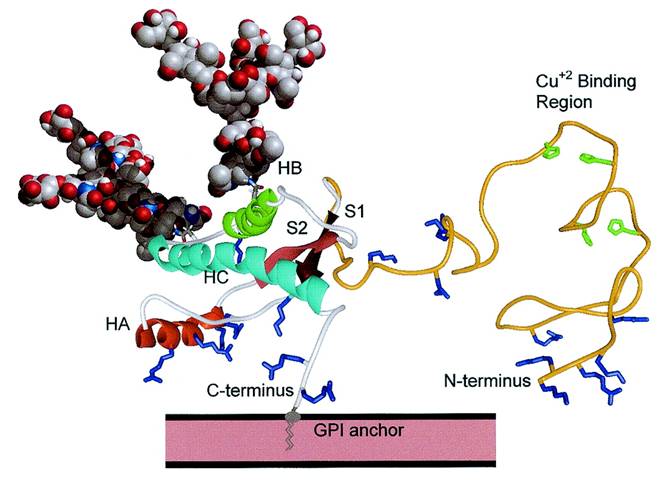
© Clinical
Chemistry -- Bennion and Daggett
(2002)
In the above NMR structure of the
normal Prion Protein Histidine residues (green) bind Cu ions, the 3 alpha helices are
shown as HA, HB and HC and the 2 Beta sheets are shown as S1 and S2. The
GPI anchor is shown attaching the Prion protein to the plasma membrane of
the cell. Lysine and Arginine residues are shown in
blue.
The normal cellular prion protein is relatively rich in alpha
helices; whereas the abnormal Isoform of the prion protein is predominantly
rich in beta sheets. The popular theory is that the abnormal Isoform of the
prion protein when it comes into contact with the cellular form induces a
conformational change in the normal form to the abnormal version of the
protein. This results in an accumulation of the abnormal Isoform to levels
dangerous to the cell.
|