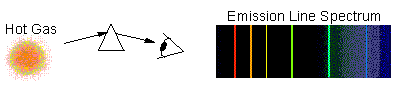Kirchhoff's Laws of Radiation
First, let's quickly review different kinds of Spectra, and how they are
created:
Continuum Spectrum
Intensity changes smoothly with wavelength
and all colors are present. This is what the spectrum of a Black
Body (such as the very hot element at the center of a light bulb)
looks like:

Emission-Line Spectrum
The light is emitted only at a few
particular wavelengths, while most of the spectrum is dark. This is typical
of hot, tenuous gas (such as in a neon-tube):
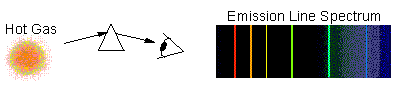
Absorption-Line Spectrum
Here we see a continuum spectrum, but it is
missing light at discrete wavelengths (they appear dark in the spectrum).
This is what occurs when a cool, tenuous gas resides in front of a bright
continuum source: