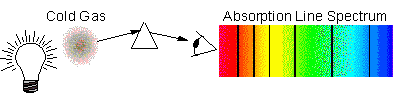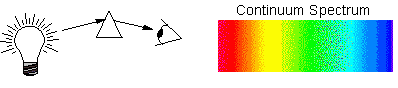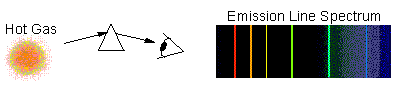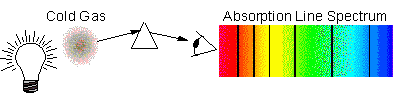Three Basic Types of Spectra
Continuum Spectrum
Intensity changes smoothly with wavelength
and all colors are present. This is what the spectrum of a Black
Body (such as the very hot element at the center of a light bulb)
looks like:
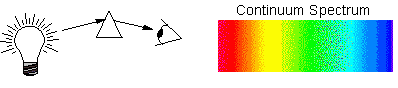
Emission-Line Spectrum
The light is emitted only at a few
particular wavelengths, while most of the spectrum is dark. This is typical
of hot, tenuous gas (such as in a neon-tube):
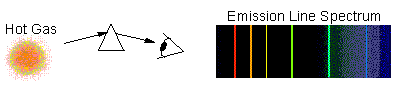
Absorption-Line Spectrum
Here, we see a continuum spectrum, but
it is missing discrete wavelengths, making them appear dark in the spectrum.
This is what occurs when a cool, tenuous gas resides in front of a bright
continuum source: