The Atmosphere and Climate on Mars
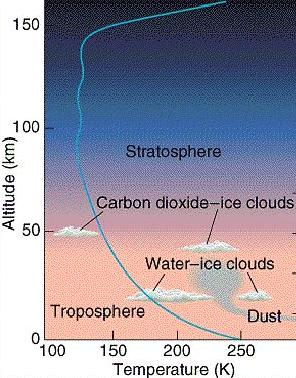
Carbon Dioxide (CO2), is the main constituent of the Martian atmosphere (95.3%) and 2.7% Nitrogen. At the very low pressures on Mars' surface, The CO2 only exists as a gas or solid (this is true on Earth, too. It's called DRY ICE! more on that soon).
Why is the pressure so low?
There is plenty of evidence that the pressure wasn't always so low.
Early volcanism would have released plenty of water and CO2 to
thicken the atmosphere. However, Mars being further from the sun,
the water might have too easily froze, and with the reduced
'greenhouse' warming of the atmospheric water, the temperature
would plumit further. Now even the CO2 condenses out of the
atmosphere at times.
The fact that the atmospheric surface pressure on Mars today is remarkably close to the "triple point" of water (below which liquid water is unstable) suggests that the atmosphere may be self-limiting.
If the atmosphere were to become thick enough for sufficient greenhouse warming to facilitate liquid water periodically at the surface, the CO2 would get removed as carbonates and tend to draw the atmosphere gradually back down to 6.1 millibars. This may be the reason for the particular value of the atmospheric pressure (and hence density) on Mars that we observe today.
