Other Unique Qualities of Water
You may not find this at all peculiar or important, but in fact it is very! Why does ice float? Solid water is less dense than liquid water. The molecules in liquid water are more densely packed together. This is because of Hydrogen Bonds.
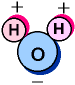
Electrons are not evenly distributed over the entire Water Molecule. In fact, the shared electrons spend far more time around the Oxygen nuclei than the H nuclei, giving it a slight charge imbalance.
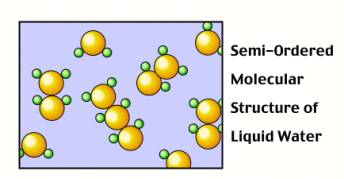
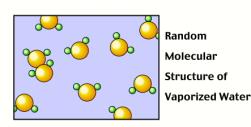
When water is in liquid or gas (vapor) form, the molecules have sufficient energy to move where they want and to arrange themselves randomly.
 |
 |
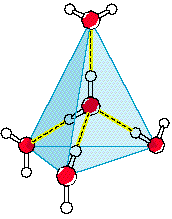
However, when water is in solid form, H-bonds begin to form: The O side of one water molecule (in red) gets sticky with the H side of another molecule (in white)! These H-bonds (shown as yellow dashes) force the molecules to arrange themselves in a very specific, way.
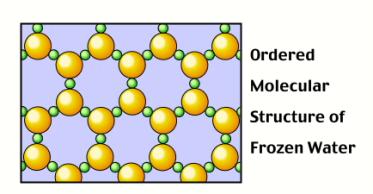
Surprisingly, the H-bonds force the solid water to arrange itself in a more spaced out orientation than when it was liquid.
Implications
More than just ice floating, it meant lakes and oceans wouldn't
freeze over! If solid water sank, then liquid water would more
easily freeze solid, from the bottom of the ocean up, killing
all living organisms contained.
What's more, without the H-bonds, water would become liquid starting
above -100 C and boil at equally cold temperatures (similar to
the other liquids we've investigated).
The Polar Nature of Water
On Earth, anyway, this is
important. Cell walls are made of long-chain non-polar
hydrocarbons, which water can not dissolve.