Other Unique Qualities of Water
C) Ice Floats!
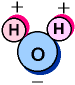
Electrons are not evenly distributed
over the entire Water Molecule. In fact, the shared electrons
spend far more time around the Oxygen nuclei than the H nuclei, giving
it a slight charge imbalance.
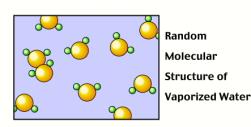
When water is in liquid or gas (vapor) form, the molecules have sufficient
energy to move where they want and to arrange themselves randomly.
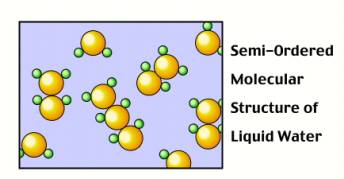
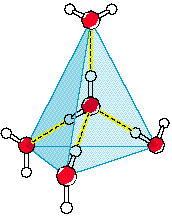
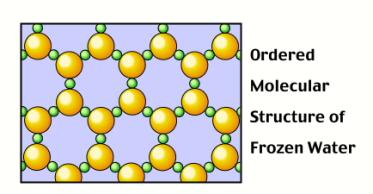
However, when water is in solid form, H-bonds begin to form: The O
side of one water molecule (in red) gets sticky with the H
side of another molecule (in white)! These H-bonds (shown as
yellow dashes) force the molecules
to arrange themselves in a very specific, way.
Surprisingly, the H-bonds force the solid water to arrange itself
in a more spaced out orientation than when it was liquid.
Implications
You may not find this at all peculiar or important, but in fact it is very!
Why does ice float? Solid water is less dense than liquid water. The
molecules in liquid water are more densely packed together. This
is because of Hydrogen Bonds.
More than just ice floating, it meant lakes and oceans wouldn't
freeze over! If solid water sank, then liquid water would more
easily freeze solid, from the bottom of the ocean up, killing
all living organisms contained.
What's more, without the H-bonds, water would freeze at
-100 C (similar to the other liquids we've investigated).