 http://www.pnas.org/cgi/content/full/100/13/7491/FIG2
http://www.pnas.org/cgi/content/full/100/13/7491/FIG2Structure/Function of PTEN
 http://www.pnas.org/cgi/content/full/100/13/7491/FIG2
http://www.pnas.org/cgi/content/full/100/13/7491/FIG2
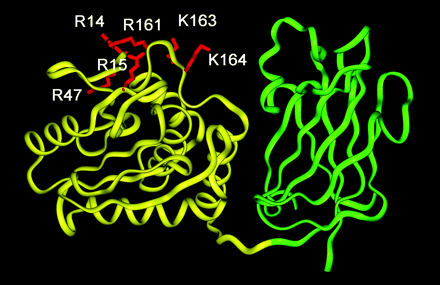
A ribbon diagram of PTEN. The phosphatase domain and the C2 domains are shown in yellow and green ribbons, respectively. The mutated residues in the phosphatase domain are highlighted in red and labeled. The molecule is oriented with its membrane-binding surface pointing upward. The coordinates are taken from the x-ray structure by Lee et al.
The PTEN structure consists of a 179-residue N-terminal domain (residues 7–185) and a 166-residue C-terminal domain . The lipid phosphatase domain is is located on the N-terminal end and the C2 domain is present on the C-terminal end. The function of the C2 domain has implications in its ability to effect cell migration through it's protein phosphatase activity in this domain. The C2 domain contains 5 phosphorylation sites. PTEN can dephosphyrlate phosphorylated proteins by accepting a phosphate from these substrates resulting in the phosphorylation of PTEN at one or more of the phosphorylation sites present in the C2 domain. Many other proteins that have domains homologous to the C2 domain have between 4-6 Ca2+ binding sites, whereas PTEN has only one. PTEN has been implicated in membrane association because of its ability to dephosphorylate PIP3, which is membrane bound. It has been shown that PTEN can associate with the membrane in a Ca2+ independent manner, via its C2 domain, in vitro.
The main physiological role of PTEN is to dephosphorylate PIP3, which antagonizes PI3 kinase activity. Downstream effectors of PI3K includes the Akt pathway, which effects cell growth and apoptosis. This effect of PTEN is exhibited through the N-terminal domain.