

Type IV secretion systems are a complex of proteins used by gram negative bacteria in order to secrete virulence factors into the environment or directly into the interior of a host cell. The evolution of this system can be traced back to the conjugal pilus used by E. coli cells for mating. This pilus allows for DNA from one E. coli bacterium to be transferred to another. E. coli conjugation movie
Many other bacteria have been identified as having a type IV secretion system. The best-studied type IV secretion system is the VirB system of Agrobacterium tumefaciens. It consists of 11 proteins that are all necessary in order to transfer a single piece of DNA into a plant cell, integrate into the plant DNA, and transforms the plant cell into a tumor. The picture on the homepage shows the structure of A. tumefaciens secretion system. The DNA is injected into the plant cell via the pilus. The pilus attaches to the host cell. Most of the other proteins involved in the system function in pilus assembly. There are several similarities between proteins in A. tumefaciens’ secretion system and B. pertussis. Both have proteins that exhibit peptidoglycanase activity. This alters the usually tightly ordered peptidoglycan layer into a loosely associated mesh and allows the larger proteins involved in the secretion of Ptx to pass through the murein layer and assemble. Both secretion systems have proteins that can bind to nTP’s, and are thought to power the secretion. The major difference between the two organisms is the way the material is secreted. A. tumefaciens directly injects its effector molecule into the target cell, while B. pertussis just releases its toxin into the environment and lets the toxin bind to the target cell and inject its effector molecule on its own.
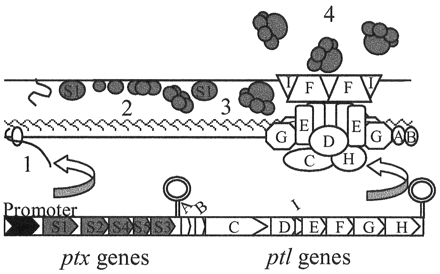
Pertussis toxin is a necessary component for colonization of the host by the bacteria. It is a classic A-B toxin, meaning that there are two major components: the B subunit that binds to the target cell and then the A subunit moves into the cell and catalyzes the ADP ribosylation of GTP-binding regulatory proteins involved in signal transduction. In order to do this, the toxin must first get out of the bacterium. The toxin and secretion system transcripts are controlled by the same promoter. Each of the toxin transcripts is passed through the peptidoglycan layer via a Sec-like system. Once past the inner membrane, the A subunit (S1) associates with the outer membrane. Then the B subunit (proteins S2-S4) combines with it, and only then will it be efficiently secreted by the secretion system. Bordetella pertussis does not have an external pilus and it does not use its secretion system for the conjugal transfer of DNA. Instead the secretion system allows for the release of pertussis toxin into the external environment. The pertussis secretion system is composed of nine different proteins (PtlA-PtlI), and these proteins come together in a complex. This model shows the proteins involved in the type IV secretion system of B. pertussis. The UFO like creatures in the diagram are pertussis toxins (Ptx). The toxin is a required element for Bordetella pertussis to successfully colonize a host organism. The type IV secretion system is necessary for the toxin to be exported from the bacteria, and the pertussis toxin must be fully assembled in order for secretion to occur. The structures for the secretion system proteins of Bordetella have not been identified, neither have the interactions between ptx and the secretion system proteins. Understanding how this system works is an area with a lot of interesting research.
 Rambow-Larson
and Alison Weiss.
Rambow-Larson
and Alison Weiss.