Type II Secretion
Type II bacterial secretion is also known as the main terminal branch of the general secretion pathway. This process occurs in a two-step process that translocates both the inner and outer membranes. Proteins that are two be secreted are produced with N-terminal signal peptides which allows for transport across the cytoplasmic membrane via Sec-dependent movement. The signal peptide is then cleaved, the protein undergoes folding, and the mature protein is released from the periplasmic space across the outer membrane. It is believed that a pilus structure helps push the secreted proteins through a gated pore. Table 2 lists some common bacteria and their properties that display this two-step process. The most commonly studied protein that uses this pathway is the cholera toxin from V. cholerae1.
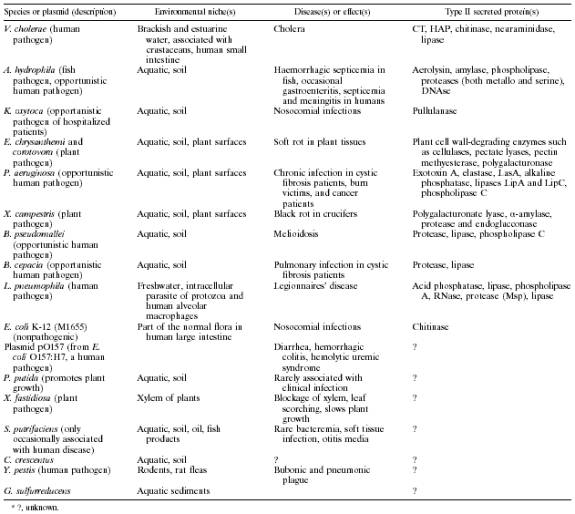
Table 2. Type II Bacteria
Secretion signals
Signals for both the secreted protein and the pili structure are found in each corresponding protein. In regards to the secreted exoprotein, it is believed that signal for secretion is created as the proteins fold inside the periplasmic space. The sequence may contain different linear arrangements that can only be recognized as the correct signal if they are brought together in the correct confirmation and was found through the use of fusion proteins3. The proper positioning of the sequence is required in order for secretion and minor structural perturbations will interfere with this process1. A couple examples of these sequences in different bacteria can be found with the enzyme pullulanase, which was found to unable to be exported across the cell membrane by removing two non-adjacent segments4.
The pilus structure contains specific steps that are adjacent to the signal peptides in order to form and assemble. Either a Lys or Arg residue needs to be cleaved off by the enzyme PilD usually 6 to 9 amino acids away from the signal sequence. The other required step is that +5 position of the pilus always contains a glutamate, which is necessary for proper N-methylation and subsequent assembly of the pilus structure5.
Mechanism/Key Proteins
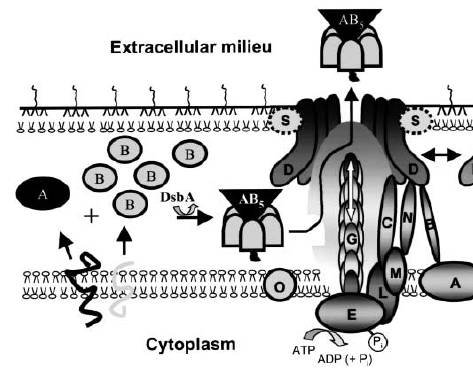
Between 12 and 15 genes have been found to be necessary for Type II secretion. The diagram below (Figure 1) outlines the important steps in the export of the cholera toxin, which becomes activated as the monomers A and B form into an AB5 complex. This assembly occurs in the periplasmic space after transport from the cytoplasm via the Sec pathway. DsbA catalyzes this assembly and allows the AB5 the Eps (type II secretion apparatus) after recognition of the B subunit that carries the secretion signal. This causes the targeting and release of the toxin. ATP hydrolysis is required to regulate and power this process. Proteins G, H, J, K, and I are processed by protein O and form the pilus structure to push the toxin through the hole. Protein S supports outer membrane insertion of protein D, protein A forms a complex in the cytoplasmic membrane with protein B, which also interacts with protein D. Not all of the mentioned proteins may be present in all bacteria that use this two-step process1.
Figure 1. Type II Cholera Toxin Export Mechanism
Regulation/Energetics
Activation or repression of the genes responsible for this
pathway occurs when diffusible auto inducers accumulate and reach a critical
concentration in the cell. Most
bacterial species use quorum sensing to control these genes through the
production and release of acylated homoserine lactones (auto inducers) that
coordinate cells in contact with each other and along with environmental
signals are both used to control expression of these proteins2.
If regulation occurs either at the transcriptional level of a virulence
factor or at the functional level of the secretion apparatus, the extracellular
secretion of the virulence factor generally occurs when the bacteria have
reached a critical mass and location, which is dependent on the species of
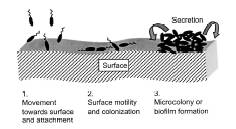
bacteria. The diagram below (Figure 2) shows
the three steps of the colonization of the surface of the host, which leads to
release of the toxins through a critical concentration via appropriate signals
from the neighboring cells, which occurs in V. cholerae2.
Figure 2. Release of the toxin
through signal activation.
The energy needed
for this process to occur usually is found from the hydrolysis of ATP. The assembly of the export apparatus and the
coupling to the transport of the exported protein seem to be linked together6, with the pilus in a continual state of
assembly and disassembly7. The energy that is provided from the
assembly of the pilus structure may also result in a physical transduction of
the energy for the export of the proteins, resulting in a biological piston or
Archimedes screw. Whatever the energy
source needed to expel the exoproteins, ATP hydrolysis or the proton-motor force
is likely to provide the necessary muscle, either indirectly through the
assembly of the pilus or through coupling of other assembly processes involved
in bacteria secretion6.