|
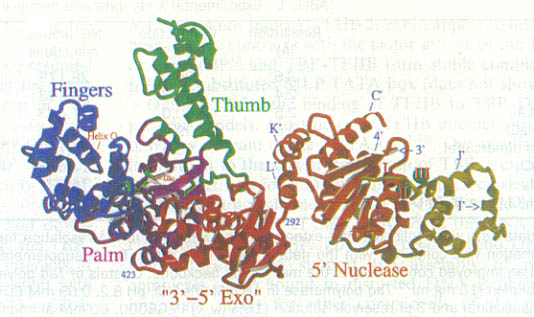
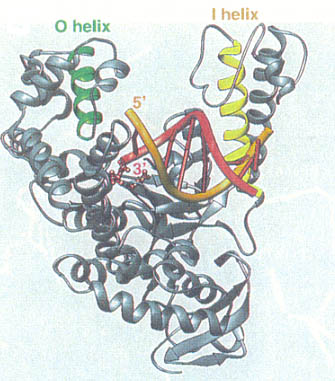
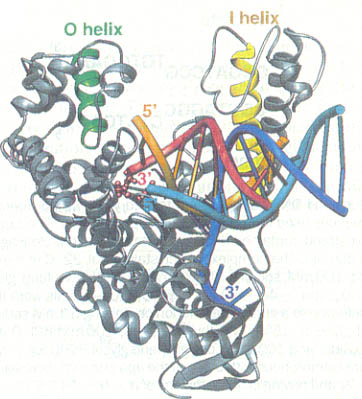
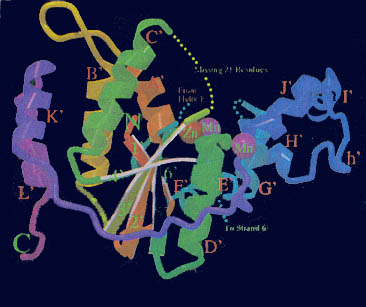
Kannan and Vishveshwara performed experiments on aromatic clusters, which they consider a determinant of thermal stability of thermophilic proteins. They used a clustering procedure, which considers a Graph Spectral method. This method detects aromatic clusters by considering all the Cβ atoms of the aromatic residues in the protein. To locate the actual aromatic residues the first step would be to use the VMD package, then calculate the solvent accessibility using Connolly’s program and lastly, graph the spectral method of assigning residues to a hydrophobic core on contact criteria. Vogt et al. correlated the importance of hydrogen bonds and salt links to stability. He measured the energy quantity of hydrogen bonds in thermostable proteins. Vogt used the HBPLUS routine, which uses donor atom-acceptor atom quantity. These different techniques can be used to compare a thermostable enzyme with a psychrophilic or mesophilic enzyme. This comparisons could determine how do hyper/thermophilic enzymes do it.
|

|
It has been possible to make recombinant Taq, using E. coli, by engineering a fusion protein along with the λgt11 library fused to a Taq library. Lawyer et al. used the LacZ gene as the reporter gene. My experimental procedures to elucidate mechanisms of stability of hyper/thermophilic enzymes: By using Lawyer et al.’s technique of actually expressing a thermophilic gene in E. coli, I can express large amount of it for further studies. By using his technique of epitope specificity, I will be able to find a specific antibody against the enzyme. I will use the kinetic experiments done by Fáigáin, who performed thermodynamic analysis of stability. I could also use the stability-activity experiments performed by D’Amico et al. by allowing the enzyme to work at different temperatures other than those at which the enzyme normally functions. He also performed unfolding-folding experiments and tested for inactivation or activation.
|