|
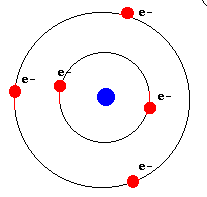
An atom is made up of three main components: protons, neutrons (found in the nucleus)
and electrons. In a neutral atom (without charge), the number of protons (positive charge)
and electrons (negative charge) are equal.
An important point is that the electrons reside in specific orbits, which circle around the central nucleus of protons and neutrons (shown here as a single blue dot). These orbits correspond to specific energies. |
|
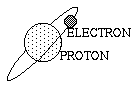
| Different elements have a different number of protons. Hydrogen has one proton (and if its neutral) one electron. This is the simplest atom in the universe. All Helium atoms have two protons, Berillium has three, and so on, building up the Periodic Table of Elements. |
|
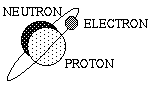
| Different isotopes of an element have the same number of protons, but differing numbers of neutrons. For instance, a common isotope of Hydrogen is Deuterium, which has an extra neutron in its nucleus. |
|
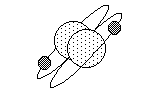
| Molecules consist of two or more atoms, bound together by atomic forces. For example, hydrogen gas occurs as a two-atom (or diatomic) molecule, as shown to the right. |
|