Life, Page 1: [Next Page] [Class Home Page]
Energy to Power Life
Life needs energy!
Energy is needed to synthesize molecules (e.g. proteins, DNA), transport
nutrients, and for processes such as locomotion. Life appears to be a direct
violation of the basic laws of Thermodynamics, but it’s not. Thermodynamics
tells us that heat flows from hot temperatures to cold. So how can a
refrigerator exist? It does so by using electricity, a form of energy. Life is
like this; it needs a continual source of energy to carry out its tasks.
Where do life forms
get their energy?
Animals oxidize their
food and plants harvest sunlight using chlorophyll. Before the energy can be
used, however, it must be transformed into a form that the organism can handle,
store, and transport easily.
 ATP = Life's Battery
ATP = Life's Battery
An important chemical in life's never ending need for energy is Adenosine
Triphosphate (ATP). It acts as a special carrier of energy.
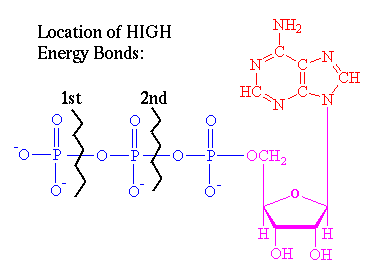
ATP is composed of three
components: In RED, the base consisting of
linked rings of C and N atoms (adenine); In PINK,
at the center is a sugar molecule (ribose, the lower right ring);
in BLUE, a string of phosphate groups
linked to the left (the most important).
When energy is
needed, the organism breaks off the 1st HIGH ENERGY bond (Creating ADP). If it
needs still more energy, it can break off the 2nd bond (creating AMP). All life
on Earth use ATP for storing and controlling their energy. Later, when the
organism is resting, the reverse reaction takes place, and the phosphate groups
are re-attached. BUT THIS REQUIRES ENERGY!!
Energy Sources
for Life
REDOX reactions: This is a combination of a reducing reaction and an oxidizing
reaction – essentially passing electrons from one molecule to another. There is
a vast number of such chemical reactions available and organisms have adapted
to use many of them.
PHOTOSYNTHESIS:
Light energy is used to create high-energy chemical bonds and to "fix" CO2
into organic carbon molecules.